
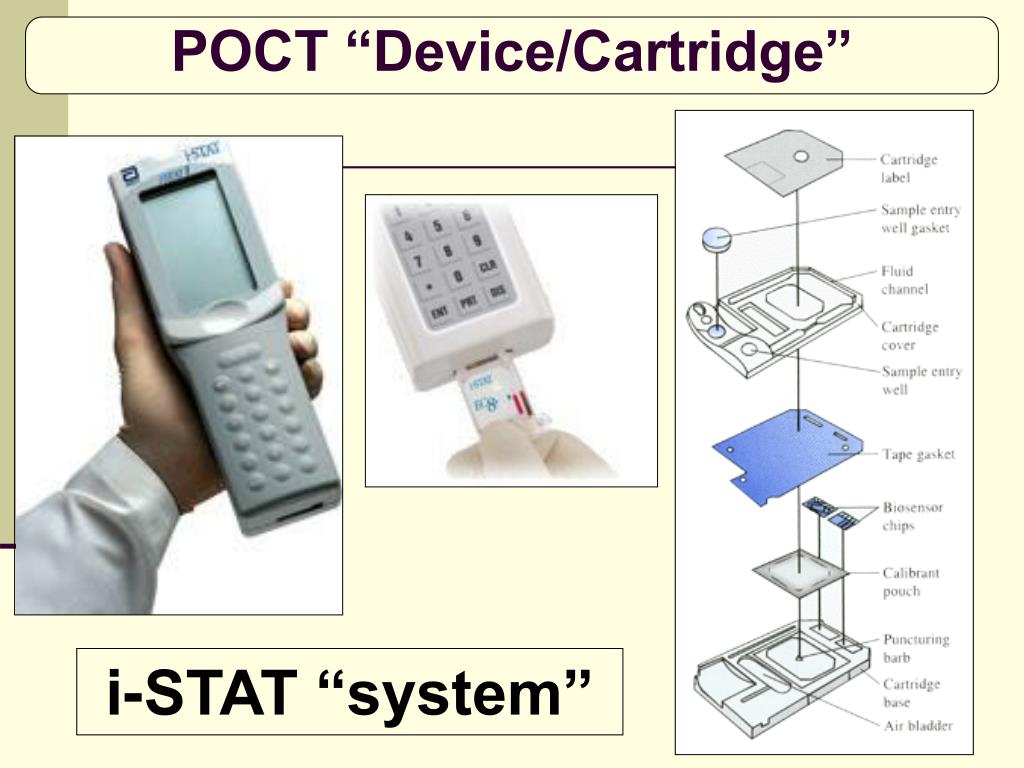

Qualitative Interpretation of Results The default setting on the handheld displays a quantitative β-hCG value as well as a qualitative interpretation of the β-hCG test result. Samples below the reportable range will display “2000.0 IU/L” on the handheld. Reportable Range The i-STAT β-hCG Test will report 5.0 to 2000.0 IU/L. Further information regarding metrological traceability is available from Abbott Point of Care Inc. i-STAT System controls and calibration verification materials are validated for use only with the i-STAT System and assigned values may not be commutable with other methods.
β-hCG values assigned to Abbott Point of Care’s controls and calibration verification materials are traceable to Abbott Point of Care’s working calibrators that are traceable to the World Health Organization’s 5th International Standard (07/364), prepared from pooled plasma and hCG antigen obtained from 3rd party sources. Metrological Traceability The i-STAT System test for β-hCG measures hCG amount-of-substance concentration in plasma or the plasma fraction of whole blood (dimension IU/L) for in vitro diagnostic use. A list of reactive ingredients is provided below: The cartridge contains a buffer and preservatives. Contents Each i-STAT β-hCG cartridge provides a sample inlet, sensors to detect the β-hCG as described above, and all the necessary reagents needed to perform the test. The electrochemical (amperometric) sensor measures this enzyme product, which is proportional to the concentration of β-hCG within the sample. The enzyme bound to the antibody/antigen/antibody sandwich cleaves the substrate, releasing an electrochemically detectable product. Within the wash fluid is a substrate for the alkaline phosphatase enzyme. The sample, as well as excess enzyme conjugate, is washed off the sensors. The hCG within the sample becomes labeled with alkaline phosphatase and is captured onto the surface of the electrochemical sensor during an incubation period of approximately seven minutes. The whole blood or plasma sample is brought into contact with the sensors allowing the enzyme conjugate to dissolve into the sample. Also deposited in another location on the sensor silicon chip is an antibody/alkaline phosphatase enzyme conjugate specific to a separate portion of the human chorionic gonadotropin molecule. Antibodies specific for β-hCG are located on an electrochemical sensor fabricated on a silicon chip. Method Explanation The i-STAT β-hCG test cartridge uses a two-site enzyme-linked immunosorbant assay (ELISA) method. β-hCG can be used for the detection of early pregnancy. TOTAL BETA-HUMAN CHORIONIC GONADOTROPIN (β-hCG) Intended Use The i-STAT® Total Beta-Human Chorionic Gonadotropin (β-hCG) assay is an in vitro diagnostic test for the quantitative and qualitative determination of beta-human chorionic gonadotropin in whole blood or plasma samples.


 0 kommentar(er)
0 kommentar(er)
